Globalization is leaving its mark on the pharmaceutical and biotech industries. While demand for medications is rising in emerging economies, cost pressures from healthcare payers in traditional markets are dampening drug makers economic prospects. The now ten-year absence of blockbuster drugs in the pipeline compounds this problem and this trend is not likely to change with current R&D processes. ARC covers top technology trends in the Life Sciences & Biotech industries on an ongoing basis. Please visit Technology Evaluation and Selection Guides page for recent ARC research rreports.

Life Sciences & Biotech Industry Overview
The current wave of mergers and acquisitions enables pharma companies to leverage new research activities, take advantage of tax-advantaged geographies, and capture new and innovative portfolios. We are also seeing an increasing trend in ?Big Pharma? for companies to selectively outsource manufacturing to contract manufacturers rather than invest in costly new production facilities. This arrangement enables branded companies to focus on core competencies in R&D and marketing while their contract manufacturing partners make the needed investments in production assets and validation. However, this also increases challenges relative to intellectual property protection.
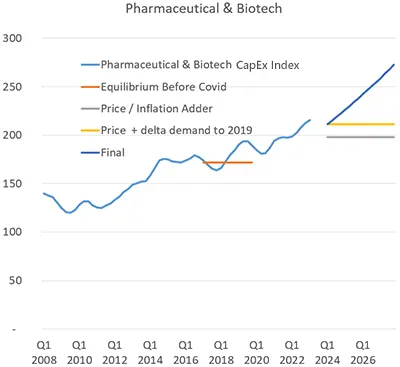 Manufacturers of branded products are operating under tighter margins due to competition from generics, pressure to reduce profits, and more regulations. While this is a business challenge, it will actually help drive capex in automation technology as companies invest in technologies to improve efficiencies, reduce time-to-market, ensure quality, and reduce costs.
Manufacturers of branded products are operating under tighter margins due to competition from generics, pressure to reduce profits, and more regulations. While this is a business challenge, it will actually help drive capex in automation technology as companies invest in technologies to improve efficiencies, reduce time-to-market, ensure quality, and reduce costs.
An important trend in pharmaceutical manufacturing is the use of modular production systems. Modularization provides the ability to scale up production of a new drug by creating multiple parallel production lines, allowing drug makers to scale up production lines in numbers but not in size.
In addition, pharmaceutical and biotech companies are investing in technologies and infrastructure necessary to achieve and demonstrate compliance with current and future global regulatory requirements, such as the US FDA?s Continued Process Verification (CPV) and 21CFR part 11. We are also seeing increased activity in pharmacogenomics, the study of a person?s response to drug therapy.
Overall, the prognosis for this industry is better than for many others thanks to two megatrends:
- Increases in global population and life expectancies
- Growing pool of middle class consumers in emerging economies who want and can now afford better healthcare.
Hindering growth are the increasing pressures on drug makers from regulatory agencies as well as fierce competition from generic drug manufacturers. That calls for more internal focus to apply technology that improves manufacturing processes with fewer internal resources.
ARC Forum Attendee Quote
"ARC's Forum is an excellent place for attendees to explore the critical issues facing our companies. As a speaker on the topic of Brand Protection and Counterfeiting and as a participant, I have benefitted from networking with industry peers. I highly recommend ARC's Forum to product manufacturers and technology users."
 Johnson & Johnson
Johnson & Johnson



