

While moving from batch to continuous manufacturing may not be new in many other industries, it is in pharmaceutical and biotech manufacturing. According to Laura Wareham, Senior Scientist, Engineering, at Merck’s manufacturing division, while continuous manufacturing may be well-established in other high-volume industries with well-known processes, it is relatively new to the pharma industry. That’s because pharma is a highly regulated, science-based, and largely batch-oriented industry with a large installed base of batch manufacturing equipment.
However, despite the upfront capital expense required to replace legacy equipment and perceived regulatory hurdles, continuous manufacturing can lead to innovative pharma and biotech products and processes and help pharma companies resolve long-standing cost issues related to development and scale-up and delivering high-quality products with flexible batch sizes to meet changing market or customer demands.
Over the past couple of years, the European Medicines Agency and US Food and Drug Administration gave a handful of approvals to pharmaceutical companies to manufacture drug products using continuous manufacturing processes. In February 2019, the FDA released a draft guidance that defined continuous manufacturing as a process in which input materials are continuously fed and transformed within the process and the output materials are continuously removed from the system.
Merck is using the OSIsoft PI System data infrastructure and piloting Seeq’s analytics to optimize its product and processes to support continuous manufacturing.
As ARC Advisory Group learned from Ms. Wareham’s end user presentation at the recent OSIsoft PI World Conference in San Francisco and subsequent discussions, Merck has initiated a project to qualify the company’s first GMP (good manufacturing practice) continuous manufacturing equipment modules and actively develop new products at the company’s facility in Cramlingon, UK. Line of site for its validation batches and filing is scheduled for later this year.
Merck’s goal for the continuous manufacturing pilot is to minimize the traditional scale-up challenges when moving from pilot production to commercial manufacturing. To reduce its equipment footprint, the company wants to be able to use the same equipment to both develop new products and produce them commercially. Compared to tradition-al batch processes, continuous processing would reduce the costs associated with scale-up and transfer between equipment and site. This ap-plies for active pharmaceutical ingredients (APIs) in particular. The project would result in flexible run times with volumes that could be adjusted to meet changing product demands. The company knew that the data-rich continuous manufacturing environment would provide opportunities to enhance both its product and process knowledge.
Merck is combining data from its OSIsoft PI System with Seeq’s analytics solution to encourage collaboration across the company and optimize its processes and products. The solution works with the company’s existing PI System data, PI Data Link, and PI ProcessBook and incorporates Seeq technology for analysis, trends, visualization, and knowledge management from the product development stage through to commercial manufacturing. The objective is to enable a highly auto-mated, agile, integrated, and optimized process.
Seeq, an OSIsoft partner, easily integrates with the OSIsoft PI System data and applications. In this application, Seeq’s advanced analytics provide decision makers with improved insights from the PI system da-ta. The PI System is the source of record. In the future, the company plans to capture process data through PI Asset Framework and PI Event Frames to enable the Seeq advanced analytics. Users will then be able to act upon the knowledge and information using PI Notifications and PI Vision.
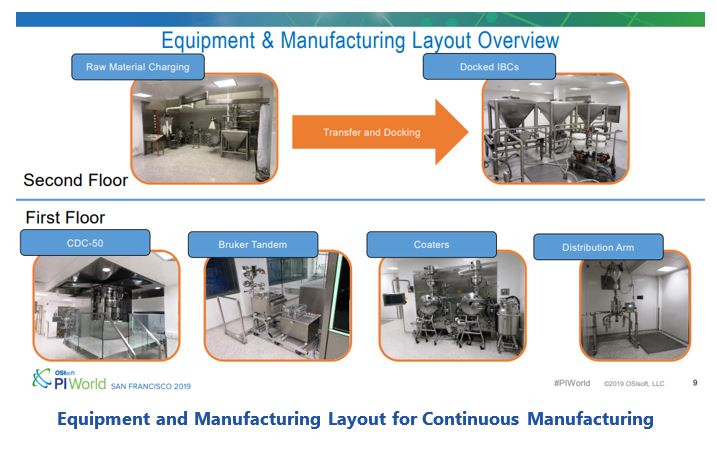
Merck’s manufacturing equipment consists of four loss-in-weight feeders that accurately dose and mix the API with three other ingredients to be mixed in the first of two linear blenders and then transported to the other linear blender and mixed with two other ingredients. This blend is then delivered to a rotary tablet press that compresses the blend into tablets. The core tablets are conveyed either to an automated tablet tester that determines tablet attributes (e.g., weight, hardness, thickness) and spectra or to a film coater. After film coating, the tablets are conveyed to a distribution arm and then discharged into finished product containers.
Merck is employing the data and analysis technology to support several use cases for converting batch manufacturing processes to continuous manufacturing as well as supporting new product development.
Traditional batch manufacturing includes multiple unit operations executed separately and sequentially. The pilot process for this tablet manufacturing application was to develop the compression or transfer of a powder or blend into a tablet in a continuous process and integrate the feeding, blending, compression and film coating operations to the upstream and downstream processes.
So far, the company has encountered no problems in compression development. Seeq can be used to analyze the relationship between multiple compression parameters as well as for upstream inputs (such as feeding parameters) and downstream outputs (such as tablet attributes). The user can quickly and easily isolate the data using Seeq’s Capsules. Once isolated, the user can review raw material characteristics and tab-let metric data using a dashboard that highlights standard deviations and process models.
The data can be filtered to remove false zero signals (e.g., when the tab-let press is off, in maintenance, or in different operation modes). This makes the data appropriate and set up for further analysis.
In a traditional batch manufacturing process, samples may be taken manually with in-process checks and then tested offline. For a representative four- hour compression run, 10 to 15 samples may be taken per batch to establish uniformity for the tablet compression. Offline testing in the analytical lab would then often take up to two weeks be-fore the batch was tested and released. For continuous manufacturing, Merck is collecting more than 1,600 tags a second.
Continuous manufacturing will generate more data in real time and enable process adjustments to be made based on trends. Innovative data analysis tools are needed to be able to take advantage of all data and to enhance visibility into the process performance. The company expects to be able to complete release testing in about a day, compared to a week for a comparable product that does not use real-time release testing.
ARC Advisory Group clients can view the complete report at ARC Client Portal
If you would like to buy this report or obtain information about how to become a client, please Contact Us
Keywords: Batch Manufacturing, Continuous Manufacturing, Analytics, Data Plat-form, Data Infrastructure, OSIsoft, Seeq, Merck, ARC Advisory Group.

