

Record New Pharmaceutical Approvals was One of the Drivers for new BMS Purchases
You can say what you will about the US government, but they have really streamlined some internal processes, such as new drug approvals, over the past couple of years. The US FDA’s Center for Drug Evaluation and Research (CDER) set an all-time record for new drug approvals (NDA) in 2018 with 59 novel drugs and biologics approved by the agency. The number of new molecular entities (NMEs) and biologics approved by CDER surpassed the agency's previous record of 53 approvals back in 1996 and is a significant jump over the 46 new drugs approved in 2017. This does not include the drugs approvals made by FDA's Center for Biologics Evaluation and Research (CBER) which are tracked separately.
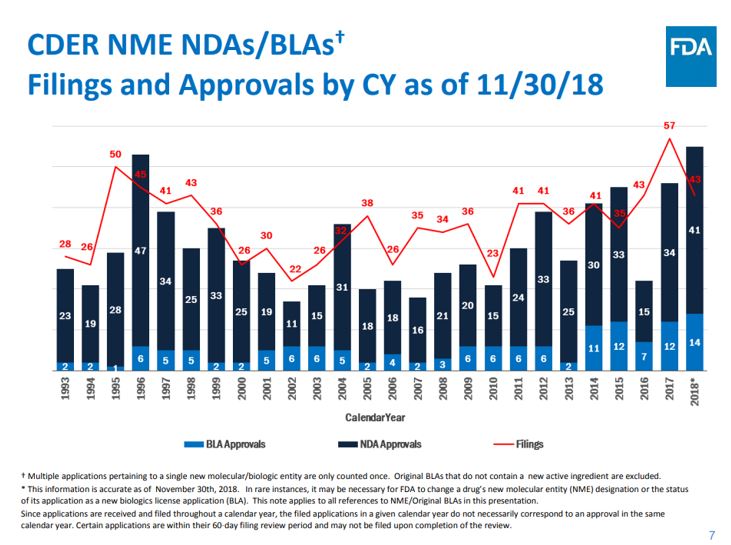
This has increased demand for new batch processes and Batch Management Software because of an increase in new production processes for the new products. Even with a push to convert batch processes to continuous processes, there was an increased demand in 2017 and 2018. Along with some other transformational drivers, as well as some newer technology drivers and enhancements, the demand for new pharmaceutical production using batch production methods, including smaller batches for personalized medicines, was one of the biggest drivers that led to an increase in demand for Batch Management Software. While many drugs are approved by different world-wide governing agencies, almost three quarters (71 percent) of the drugs in 2018 were first approved in the United States, which is still the largest market for prescription drugs, with about 45 percent market share worldwide. Thus, the reason for the push for modern Batch Management Software.
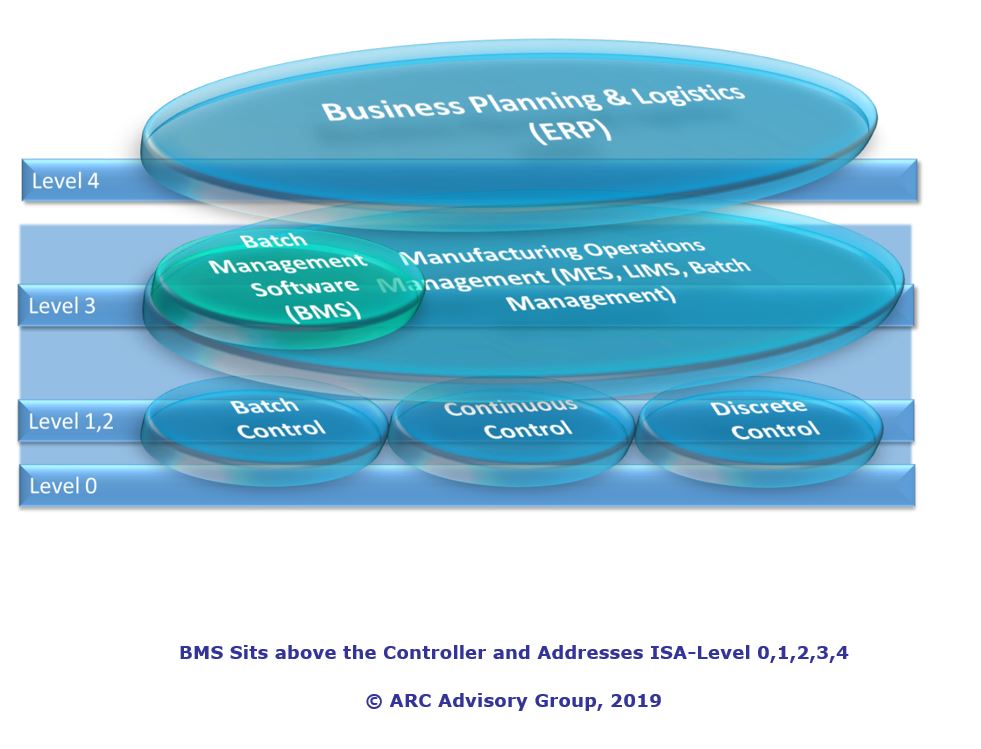
Batch Management Software technology can help drive faster and smoother startups and shutdowns. The technology enables more efficient batch processes, improves product quality and batch-to-batch consistency, reduces processing errors, streamlines compliance reporting and ultimately optimizes batch production operations. All of which make it possible to produce products more efficiently for less cost in all batch industries. ARC believes that an increased pharmaceutical approval trend, new technology enhancements, and the importance of speed-to-market will increase in Batch Management Software purchases into 2019 and over the forecast period 2019 to 2023.
For additional information about the BMS market and technology trends and forecast through 2023, see the recently completed market research on this topic. See What is Batch Management Software, ARC’s Batch Management Software Global Market Research and/or ARC’s Batch Management Software Selection Guide.

